
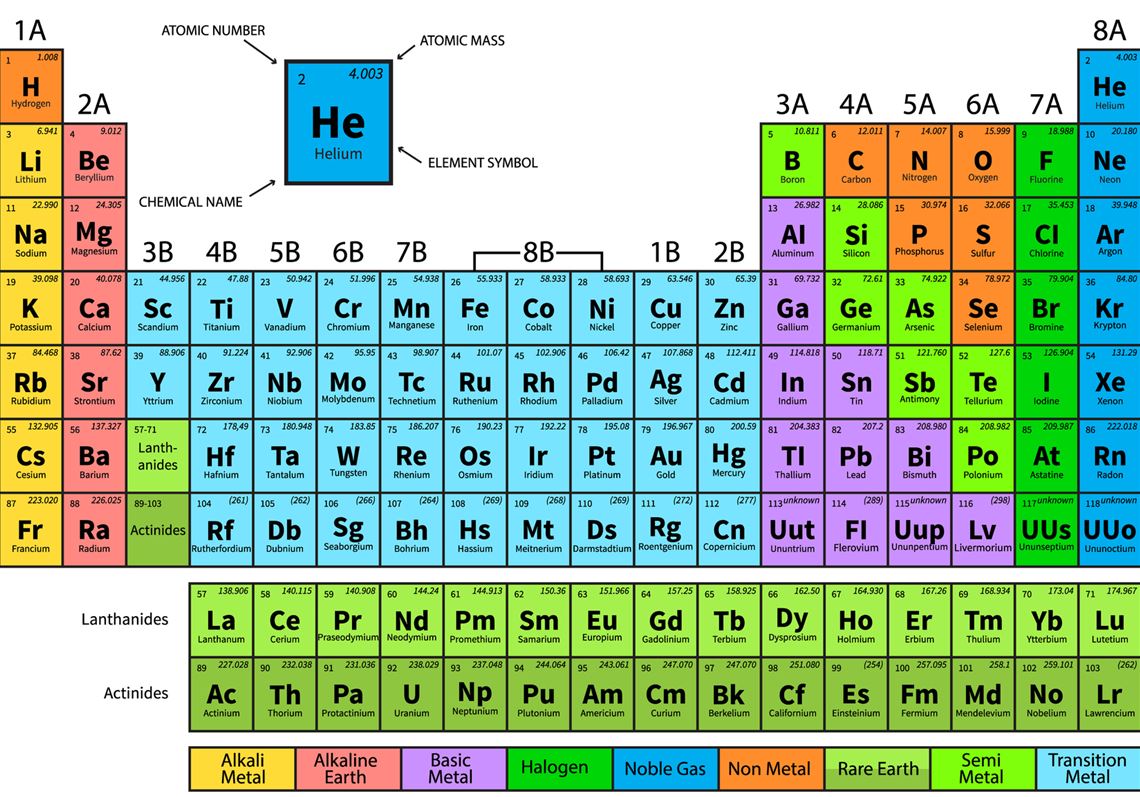
USCP: Indicates that credit in the course satisfies the U.S. GE Area: Indicates the General Education (GE) Area for which the course may fulfill a requirement. Term Typically Offered: F = Fall quarter, W = Winter quarter, SP = Spring quarter, SU = Summer quarterĬR/NC: Indicates a course is offered on a Credit/No Credit grading basis only. The periodic table explains the number of protons, neutrons, and electrons, the mass, atomic number, mass number, valence electrons, etc.The bolded first line begins with a capitalized abbreviation that designates the subject area followed by the course number and title. It is to be noted that while moving from left to right across a period, the more we escalate towards the right, the more energy is required to remove an electron because they become more tightly bound. This happens because each successive element has an added electron and proton. Atomic radius tends to decrease as we move left across a period. Periodic numbers are the numbers placed beside the periodic table. Elements in the same group have the same number of electrons which shows that they have similar properties. Group numbers are placed above the periodic table. The letters in each block represent the atomic symbol. The number of protons and electrons in the element are indicated by the atomic number. Each element is placed in its specific location due to its atomic structure. Each row and column have specific characteristics. It is color-coded for easier reference by students and researchers. The periodic table of elements includes names, atomic number, symbol and mass.
Also known as the periodic table of elements, the organization is such that it is easy for any layman who has basic knowledge of chemistry to understand the unique properties of individual elements. Periods have metals on the left and non-metals on the right. There are seven rows in the table, termed as periods. The periodic table is a tabular display of chemical elements arranged based on the atomic number, electron configuration, and chemical properties. Special chemistry tuition is conducted to give a clearer understanding of the module.

From the very beginning, the table is explained for the students to understand the concepts better. Russian chemist Dmitri Mendeleev published the first recognizable periodic table in 1869. The periodic table is regarded as the most important aspect of chemistry. In our dynamic classes of A level chemistry tuition, O level chemistry tuition, JC chemistry tuition for the students of Singapore, our teachers will show you how impressive Chemistry can be and will guide you to success. They often think that Chemistry is a subject which is only about memorizing the various complicated laws and principles. We believe that this is because of the myths that develop inside various students while studying Chemistry. Most of the time it has been branded as a very bland and boring subject to read. Chemistry has never been very successful in attracting students.


 0 kommentar(er)
0 kommentar(er)
